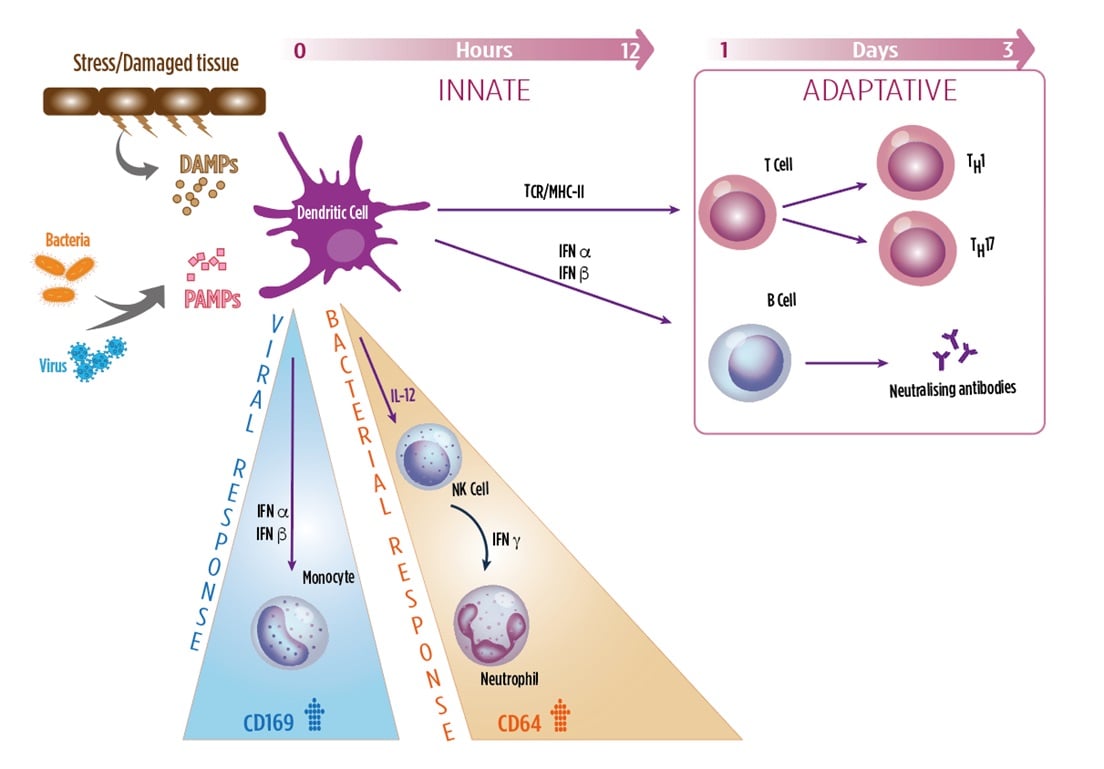
Innate versus Adaptive Immune Response
Myeloid cells, such as granulocytes, monocytes, NK cells, DCs, contribute to the innate immune system in recognizing invading pathogens and tissue damage. These cells are activated upon contact with microbial and damage patterns (PAMPs, DAMPs) triggering a robust inflammatory response and release various pro-inflammatory mediators such as cytokines, interferons (IFNs) and specific cellular markers. This constitutes the immediate response to infection, provided by the innate immune system.
Adaptive immunity, which is required for the long-term, sustained response to infection, is activated through the classical MHCI and II/TCR interaction with activated dendritic cells. Interferons also act as a key link between the innate immune response and activation of the adaptive immune response.
CD64 (FCγ-RI)
- Provide a first line of recognition and defense against infections
- Bacterial infections, leads to release of type II IFN (IFNγ), which strongly induces the expression of CD64 on neutrophils.
CD169 (Siglec-1)
- Adhesion receptor, recognizing sialylated glycoproteins and glycolipids of viral membranes
- Viral infections, leads to release of type I IFNs (IFNα, β), which strongly induces expression of CD169 on monocytes
HLA-DR
- MHC-Class II receptor mainly involved in viral antigen presentation to T cells
- HLA-DR on monocytes is induced immediately after infection, slowly declining with exhaustion.
References:
- Crocker PR. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med. 164(6), 1862–1875 (1986).
- Macauley MS, Crocker PR, Paulson JC. Siglec regulation of immune cell function in disease. Nat. Rev. Immunol. 14(10), 653–666 (2014).
- Pino M, Erkizia I, Benet S, et al. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 12(1), 1–15 (2015).
- Kim W-K, McGary CM, Holder GE, et al. Increased Expression of CD169 on Blood Monocytes and Its Regulation by Virus and CD8 T Cells in Macaque Models of HIV Infection and AIDS. AIDS Res. Hum. Retroviruses 31(7), 696–706 (2015).
- Bourgoin P, Biéchelé G, Ait Belkacem I, Morange PE, Malergue F. Role of the interferons in CD64 and CD169 expressions in whole blood: Relevance in the balance between viral- or bacterial-oriented immune responses. Immun Inflamm Dis. 2020;8(1):106-123. doi:10.1002/iid3.289.
- Kipfmueller F, Schneider J, Prusseit J, et al. Role of Neutrophil CD64 Index as a Screening Marker for Late Onset Sepsis in Very Low Birth Weight Infants. PLoS ONE 10(4), 1–15 (2015).
- Selvaraj P, Fifadara N, Nagarajan S, Cimino A, Wang G. Functional Regulation of Human Neutrophil Fc γ Receptors. Immunol. Res. 29(1–3), 219–230 (2004).
- Krensky, A.M. The HLA system, antigen processing and presentation. 1997, Kidney International, suppl. 58, 51, 2-7.
- Lee, J., Dupont, B.O. The HLA system: An introduction. 1990, "The HLA system: A new approach", Springer-Verlag, 1-26.
- Uckun, F.M. Regulation of human B-cell ontogeny. 1990, Blood, 76, 1908-1923.
- Kontny, E., Ryzewska, A. Surface markers on human activated T lymphocytes IV. Comparison of high-affinity E-rosette receptor expression with the expression of other activation markers (receptor for Interleukin 2, MHC class II (antigens). 1990, Archivum Immunologiae et Ther. Experimentalis, 38, 421-431.
- Venet F, Lukaszewicz AC, Payen D, Hotchkiss R, Monneret G. Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Curr Opin Immunol. 2013;25(4):477-483. doi:10.1016/j.coi.2013.05.006.

